Concentration Cell with Transference
Concentration Cell with Transference
When two solutions are in direct contact in galvanic cell, then the transfer of ions occurs from one to other solution directly is called concentration cell with transference.
Let us consider the following concentration cell with transference-
Pt, H(g) HCl(a1) | HCl(a2) | H2(g), Pt
On passing one faraday of electricity through this cell, one g- atom of hydrogen gas is oxidized to one g- ion of H+ ions at the left electrode and the same amount is reduced to produce one g- atom of hydrogen gas at the right electrode. If t+ and t- are the transport number of H+ and Cl- ions, then during the passage of one faraday of electricity through the cell, t+ faradys will be carried by t+ g- ions of H+ ions in one direction while t- faradays will be carried by t- g- ions of Cl- ions in the opposite direction-
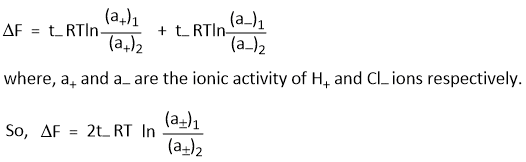
The net result is an increase in left electrode of 1 - t+ = t- g- ion of H+ ions and t- g- ion of Cl- ions at the same time a decrease of 1 - t+ = t- g- ions of H+ ions and t- g- ion of H+ ions and t- ion of Cl- ions in the right electrode. This is equivalent to the transfer of t- g- ion of Cl- ions from right to left. Hence, free energy change accompanying the transfer,
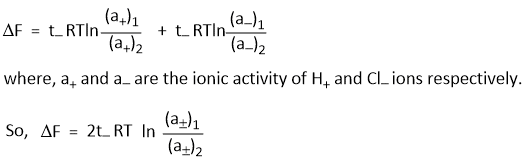
Where (a±)1 and (a±)2 are the mean ionic activities in two HCl solutions.
ΔF = −FE for one mole,
Hence emf of the concentration cell with transference-
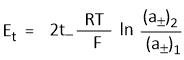
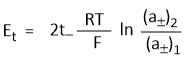
In other words, emf of such cells depends on the transport number of anion and the ratio of the mean activities. Similarly, if electrodes are reversible with respect to the anion then-
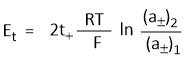
The emf include the liquid junction potential (ELJP) between the two HCl solutions.
If H+ and Cl- ions have the same ativity in a given solution, then a+ = a- and the emf of the cell in which EL is eliminated would become-

This equation holds good for univalent electolytes.
Again- t+ + t− = 1
or, t− − 1 = −t+
on adding t− both sides, we get-
t+ + (t− − 1) = t− − t+
or, 2t− − 1 = t− − t+
Putting the value of (2t− − 1) in the above equation, we get-
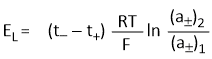
The sign and magnitude of EL depends upon the transport number of cation and anion.
If the transport number of ions are not very different, EL will be small. This explains the use of concentrated solution of KCl to minimise ELJP as the transport numbers of K+ and Cl− ions are almost equal.
