Liquid Junction Potential
Liquid Junction Potential
When two electrode solutions of different concentrations are placed in close contact, then ions diffuse from higher to lower concentrations. The rate of diffusion is almost proportional to the speed of the ions in the electric field. So if cation moves faster, then cation diffuses quickly in dilute solution than the anion and hence the dilute solution gets a positive charge.
Similarly, if anion moves faster, then the dilute solution gets a negative charge. In either case, an electrical double layer is set up at the junction of two solutions and thus a potential difference is developed at this junction.
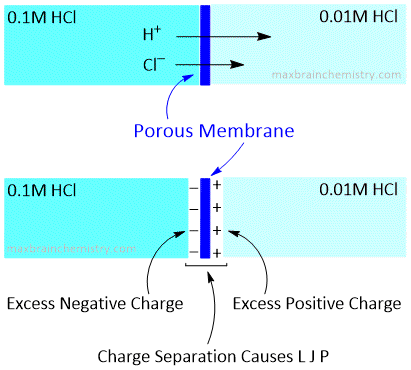
The potential difference developed at the junction of two solutions of different concentrations is called liquid junction potential. The liquid junction potential arises due to the different migrational velocities of two ions and its magnitude depends upon the relative speed of two ions. In case the two ions have the same speed, then there will not be any liquid junction potential.
The liquid junction potential interferes with the exact value of the emf of a chemical cell. It should be minimize as much as possible. To eliminate this liquid junction potential, salt bridge is used where an electrolyte (KCl, NH4NO3, CH3COOLi) is used in large amount between the two solutions constituting the junction.
Elimination of liquid junction potential
In order to eliminate or minimize the liquid junction potential, a salt bridge (consisting usually of a saturated solution of KCl or KNO3 or NH4NO3) is placed between the two solutions. The liquid junctions potential depends upon the concentration of KCl or KNO3 which is present in the salt bridge.
Why is a salt bridge (acts as semipermeable membrane) used to eliminate liquid junction potential?
A salt bridge is used to eliminate the liquid junction potential in electrochemical cells because it allows for the flow of ions between the two half-cells, preventing the development of a potential difference at the interface between the two electrolyte solutions. This helps maintain electrical neutrality and ensures accurate measurements.
