Critical Solution Temperature
Critical Solution Temperature or Consulate Temperature of System
A large number of liquids are only partially miscible with each other. Two solutions of partially miscible liquids, which are in equilibrium are called conjugated Solutions at a particular temperature. When equal volumes of ether and water are shaken together, two layers are formed, one of a saturated solution of ether in water and the other of a saturated solution of water in ether. These two solutions are referred to as conjugate solutions. The two layers of a conjugated solutions become completely miscible (homogeneous) at a certain temperature whose value depends upon the nature of the two liquids.
The temperature at which two partially miscible liquids become completely miscible at all proportions is called the Critical Solution Temperature (CST) or consulate temperature of the system.
When the critical solution temperature is lower than the room temperature, it is known as Lower Critical Solution Temperature which may be achieved by lowering the temperature of the system. On the other hand, when the critical solution temperature is higher than the room temperature, it is known as Upper Critical Solution Temperature which may be achieved by raising the temperature of the system.
Depending upon the Critical Solution Temperature, partially miscible systems are categorized into three classes. They are-
1. Type I: Partially miscible systems with Upper critical solution temperature (UCST).
Example- Phenol - Water system
2. Type II: Partially miscible systems with Lower critical solution temperature (LCST).
Example- Triethylamine - Water system
3. Type III: Partially miscible systems with Upper CST and Lower CST.
Example- Nicotine - Water system
Upper Critical Solution Temperature (UCST)
The upper critical solution temperature (UCST) or upper consolute temperature is the critical temperature above which the components of a mixture are miscible in all proportions.
Phenol- Water System
When almost equal amounts of phenol and water are mixed two layers are produced. Viz. a solution of phenol in water and a solution of water in phenol. The composition of each of these layers is fixed at any given temperature and can be determined by the analytical methods.
The curve in Figure represents the miscibility of phenol and water. The left hand side of the parabolic curve represents one of the two conjugate solutions which depicts the percentage of phenol dissolved in water at various temperatures. The solubility of phenol increases with temperature. The right hand side of the curve represents the other conjugate solution layer that gives the percentage of water in phenol. The solubility of water in phenol also increase with increase of temperature.
The two solution curves meet at the maxima on the temperature-composition curve of the system. This point here corresponds to temperature 66°C and composition of phenol as 33%. Thus, at a certain maximum temperature, the two conjugate solutions merge, become identical and only one layer results. The temperature at which the two conjugate solutions (or layers) merge into one another to from one layer, is called the Critical Solution Temperature (CST) or Upper Consolate Temperature. This is characteristic of a particular system and is influenced very much by the presence of impurities. The determination of critical solution temperature may, therefore, be used for testing the purity of phenol and other such substances.
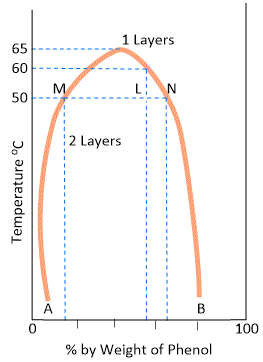
At any temperature above the critical solution temperature, phenol and water are miscible in all proportions. Outside the curve there is complete homogeneity of the system, i.e., one layer only exists; and under the curve there may be complete miscibility but it depends upon the composition of the mixture. It is clear from the above figure that at a temperature below 50°C a mixture of 90% phenol and 10% water or 5% phenol and 95% water, will be completely miscible since the corresponding points do not lie under the curve. Two layers will always separate out below the curve and the curve gives compositions of the conjugate solutions constituting the two layers. At 50°C a mixture of equal proportion of phenol and water (50% each) will form two layers whose compositions are given by A and B. The line joining the points (M and N) corresponding to the compositions A and B is called the tie line. This line helps in calculating the relative amounts of the two layers, which is here given by the ratio MN/ML.
Some other liquid pairs behaving like phenol-water system are given below with their CST values and the percentage of the first component being given in bracket.
a. Methanol-Cyclohexane (49°C; 29)
b. Hexane-Aniline (59.6°C; 52)
c. Carbon disulphide-Methanol (49.5°C; 80)
Lower Critical Solution Temperature (UCST)
Triethylamine - Water System
The lower critical solution temperature (LCST) or lower consolute temperature is the critical temperature below which the components of a mixture are miscible in all proportions.
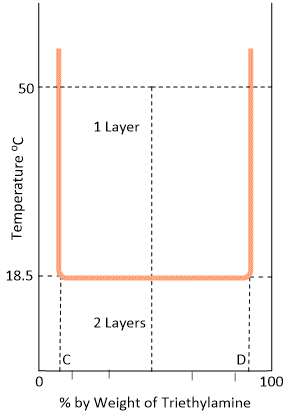
The temperature-composition curve of mutual solubilities of triethylamine and water is given in the Figure. The left hand side of the curve indicates the solubility curve of triethylamine in water and the right hand side of that of water in triethylamine. Unlike phenol-water system, the solubilities decrease with the increase in temperature in this system. The two conjugate solutions mix up completely at or below 18.5°C. This temperature is also called the critical solution temperature or the lower consolate temperature. As in the above case, any point above the horizontal line corresponds to heterogeneity of the system (two layers) while below it is complete homogeneity (one layer). Thus an equi-component mixture (50–50) will be completely miscible at 10°C but at 50°C there will be separating out two layers having compositions corresponding to the points C and D.
Common examples of this system with their lower critical solution temperatures and percentage of the first component are given below.
a. Diethylamine-Water (43°C; 13)
b. 1-Methylpiperidine-Water (48°C; 5)
Upper and Lower Critical Solution Temperature (ULCST)
Nicotine - Water System
The behaviour of this type of system is as if it were a combination of the first two types. At ordinary temperature nicotine and water are completely miscible but at a higher temperature the mutual solubility decreases and as the temperature is raised further the two liquids again become miscible. In other words, the mutual solubility increases both on lowering as well as raising the temperature in certain ranges.
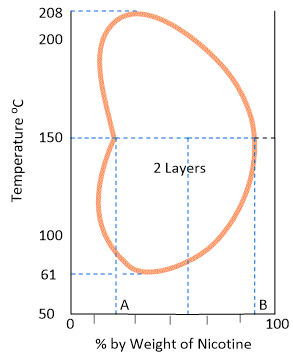
Thus, we have a closed solubility curve and the system has two critical-solution-temperatures, the upper 208°C and the lower 61°C. The effect of pressure on this system is that the lower critical temperature is raised while the upper critical temperature is lowered gradually until
finally they become one. At this point the liquids are miscible at all the temperatures.
Glycerine – m-Toluidine, and Water – β-Picoline are other examples of this type.
Effect of Impurities on Critical Solution Temperature
Critical solution temperature can be affected by impurities present in the mixture. The presence of impurities can cause a shift in the Critical Solution Temperature in different directions depending on the nature of the impurities.
Shift in the Critical Solution Temperature to a Higher Value
The presence of impurities that have a higher boiling point than the components in the mixture can cause the Critical Solution Temperature to shift to a higher value because the impurities act as a barrier to the mixing process and increase the energy required for the components to mix completely. The impurities cause an increase in the intermolecular forces between the components, leading to an increase in the Critical Solution Temperature.
Shift in the Critical Solution Temperature to a Lower Value
The presence of impurities that have a lower boiling point than the components in the mixture can cause the Critical Solution Temperature to shift to a lower value because the impurities can act as a catalyst for the mixing process, reducing the energy required for the components to mix completely. The impurities cause a decrease in the intermolecular forces between the components, leading to a decrease in the Critical Solution Temperature.
No effect on Critical Solution Temperature
In some cases, the impurities present in the mixture may not have any effect on the Critical Solution Temperature. This can happen if the impurities have similar properties to the components in the mixture, such as molecular size and shape. In such cases, the impurities do not affect the intermolecular forces between the components, and the Critical Solution Temperature remains the same.
In which system both upper and lower critical solution temperature is found —
A. Water-Triethylamine systemB. Water-Nicotine system ✔
C. Water-Phenol system
D. Water-Aniline system
Critical solution temperature (or the con-solute temperature) for partially miscible liquids (e.g., phenol-water) is the minimum temperature at which
A. A homogeneous solution (say of phenol water) is formed ✔B. Mutual solubility of the two liquids shows a decreasing trend
C. Two liquids are completely separated into two layers
D. None of these
