Concentration Cell without Transference
Concentration Cell without Transference
The chemical cell in which two electrolyte solutions are not in direct contact with each other and the transference of ions from one solution to other does not take place directly. The electrolytes are connected by salt bridge.Let us consider a chemical cell having hydrogen electrode and Ag-AgCl electrode dipped in a1 molar HCl solution.
Pt, H2(g) | HCl(a1), AgCl(s) | Ag
The cell reaction is given as-
1/2 H2(g) + AgCl(s) = H+(a+)1 + Cl-(a-)1 + Ag(s)
where, (a+)1 and (a-)1 are the activities of H+ and Cl- ions in HCl solution of activity a1.
If the same cell is reversed when activity changes to a2-
Ag | AgCl(s), HCl(a2) | H2(g), Pt
Then the cell reaction becomes-
H+(a+)2 + Cl-(a-)2 + Ag(s) = 1/2 H2(g) + AgCl(s)
When these cells are connected so as to oppose each other, then the overall cell is given as-
Pt. H2(g) | HCl(a1), AgCl(s) | Ag-Ag | AgCl(s),HCl(a2) | H2(g),Pt
The overall reaction for the passage of one faraday is given by-
H+(a+)2 + Cl-(a-)2 = H+(a+)1 + Cl-(a-)1
Thus, for the flow of one faraday of electricity, the overall reaction is the transfer of one mole of each of H+ and Cl- ions or one mole of HCl, from a solution of activity a2 to that of activity a1.
Hence, the free energy change accompanying the transfer is given as-
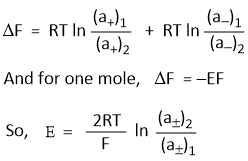
Where (a±)1 and (a±)2 are the mean ionic activities in two solutions. A cell of this type is called concentration cell without transference in which the emf depends on the relative activities of two solutions.
Note:
The transfer takes place from more concentrated solution to less concentrated solution creating potential difference at the equilibrium.
For the feasibility of the reaction, a2 > a1 means the EMF of RHS cell must be more than LHS electrode.
When a1 = a2 , the reaction cease & EMF becomes zero.
If the middle electrode Ag, AgCl(s) is removed, the two HCl solution will come in contact with each other and becomes concentration cell with transference.
Concentration without transference contains 3 electrodes: 2 are reversible wrt cation (H+) & other is reversible wrt anion (Cl-)
Concentration Cell with Transference
Share
