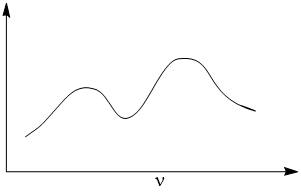
IR spectra of Fe2(CO)9 shows two νCO bands. Explain.
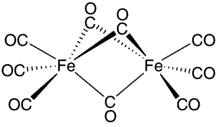
Fe2(CO)9 has two types of CO groups- three bridging and six terminals. Terminal CO groups have bond order about three and bridging CO groups have bond order about two. The bridging CO behaves as ketonic group in inorganic compounds. Hence, terminal and bridging CO groups appears at 2425-1850 cm− and below 1760 cm− respectively. Due to higher bond order, terminal CO appears at higher frequency in comparision to bridging CO. Therefore, Fe2(CO)9 shows two νCO bands.
Another important band in the IR spectrum of Fe2(CO)9 is the metal-metal stretch band, which appears at around 550-600 cm−. This band results from the stretching vibration of the Fe-Fe bond and is a strong indication of the dimeric structure of the compound. The intensity and position of this band can be affected by the nature of the ligands present and the coordination geometry around the iron atom.