Applications or Significance of Dipole Moment
Dipole moment play an important role in understanding the nature of chemical bonds and the overall polarity of molecules. They provide valuable insight into the distribution of charge within a molecule, which in turn affects its physical and chemical properties. Some important Applications of dipole moment are dscussed below-
Determination of Bond Moment
The contribution of individual bond towards dipole moment of polyatomic molecule is called bond moment as the dipole moment is the vectorial sum of individual bond moments. The dipole moment of water is 1.85D,

Hence, the bond moment of the O—H bond in H2O is calculated by using Lami's theorem of three coplanar forces in equilibrium as-

Determiation of % ionic character of a Bond
Polar compounds have ionic as well as covalent character. The ionic character arises due to polarity in bond. The ionic charater can be calculated from the experimently detrmined dipole moment.
In H-Cl, bond distance is 1.26Å and dipole moment is 1.03D. If the charge(δ) be 1unit then, the dipole moment(μ) will be-
μcal = 4.8 x 10−10 x 1.26 x 10−8 cm = 6.05D.
But the observed(expermintal) value of H-Cl is 1.03D. Hence, the % ionic character of H-Cl bond will be-
[μobs/μcal] x 100 = 1.03D/6.05D = 17%.
This means that the H-Cl is only 17% ionic and 83% covalent.
Ionic character increases as the electronegativity difference between the bonded atoms increases. Thus HF is most ionic and HI is least ionic among the HX molecules.
Distinction of cis-trans Isomers
In most of the cases, dipole moment of cis-isomer has higher value than the more symmetrical trans isomer because, cis isomer of the molecule is quite polar as the bonds are on the same side of the molecule and don't cancel out completely. So the overall molecule is polar and dipole moment is more.

Examples-
Dichloroethylene
cis-isomer: 1.56D
trans-isomer: 0.3D
1,2-dichloroethene
cis-isomer: 1.86D
trans-isomer: 0D
1,2-dichloroethene
cis-isomer: 1.86D
trans-isomer: 0D
1,2-dibromoethene
cis-isomer: 1.35D
trans-isomer: 0D
Exception: In Cyclooctene, the trans isomer has more dipole moment (0.8D) than that of cis isomer (0.4D) due to the more symmetrical arrangement of bonds in the trans isomer.

Determination of Structure of Molecules
Structure of a molecule can be determined by the dipole moment. All the symmetrical molecule have zero dipole moment. All the monoatomic molecules are symmetrical because their dipole moment is zero. Homonuclear diatomic molecules have zero dipole moment hence they are symmetrical linear molecules. The heteronuclear diatomic molecules are also linear, though they do have dipole moment.
AB2 type molecule can have either linear or angular structure.
example- CO2 and H2O is a AB2 type molecule, CO2 is linear while H2O is angular because the dipole moment of CO2 is zero while that of H2O is 1.85D.
Dipole moment of CCl4 is zero, so, it's structure is symmetrical tetrahedral.
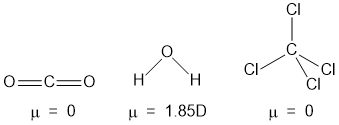
Molecules having completly symmetrical structure don't have dipole moment. Examples are- CO2, CS2, C2H4, CCl4, SnCl4 etc.
Distribution between ortho, meta and para-Isomers
Applying the law of parallelogram of forces in the following cases, we get-

For ortho-isomer:
R2 = P2 + Q2 + 2PQ cosθ
or, R2 = P2 + P2 + 2P2 x 1/2 = 3P2
∴ R = √3P = √3 x 3.9 = 1.732 x 39 = 6.63D
μcal = 6.63D
μobs = 6.00D
For meta-isomer:
R2 = P2 + P2 + 2PQ cosθ
or, R2 = P2 + P2 + 2P2 x (−1/2) = P2
∴ R = P + 3.90D
μcal = 3.90D
μobs = 3.90D
For para-isomer:
R2 = P2 + P2 + 2PQ cosθ
or, R2 = P2 + P2 + 2P2 x (−1) = 0
∴ R = 0
μcal = 0
μobs = 0
NO2 group has dipole moment 3.9D. The difference in μ values of ortho-isomer is due to mutual repulsion between two NO2 groups. Hence, the actual bond angle is greater than 60°.
Explanation of the behaviour of Solutions and Gases
Dipole moment is quite helpful in the interpretation of behaviour of solutions and gases which is often different from their ideal states.
Explanation of solubilities
Study of dipole moment is quite valuable in explaining the solubilities of substances. Polar molecules are more soluble in polar solvents like water, while nonpolar molecules dissolve better in nonpolar solvents like oil.
Influence of Solvent
Dipole moment is very helpful in explaining the influence of solvents in reaction rates.
Boiling and Melting Points
Polar molecules have higher boiling and melting points due to stronger intermolecular forces (dipole-dipole interactions).
Chemical Reactivity
Polar molecules are more reactive than nonpolar molecules due to their ability to form bonds through electrostatic interactions.
Spectroscopic Analysis
Dipole moment plays a very important role in spectroscopic techniques like infrared (IR) and Raman spectroscopy. By measuring the changes in dipole moment during molecular vibrations, these techniques can identify and characterize functional groups within a molecule.
