Quenching of Orbital Angular Momentum
Orbital angular momentum arises due to the rotation of orbitals. It has been found that orbital angular momentum is partially reduced even in fee ion and in crystal field.
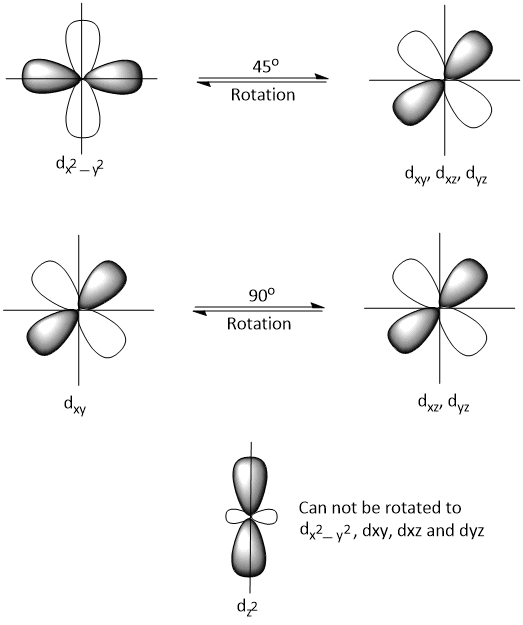
In free ion, dxy, dxz and dyz orbitals can rotate by 90° to each other and dx2−y2 can not be rotated by 45° to dxy, dxz and dyz and vice-versa but dz2 can not be rotated to dx2−y2 or dxy, dxz and dyz. Thus, one fifth of the orbital angular momentum is reduced or quenched even in free ion.
In the crystal field, d-orbital splits as-

The energy of dx2−y2 and dxy, dxz and dyz orbitals are differ in crystal field and so, dx2−y2 can not be rotated to dxy, dxz and dyz and vice-versa to give orbital of equivalent energy. Hence, in the crystal field, there is a further reduction or quenching of orbital angular momentum.
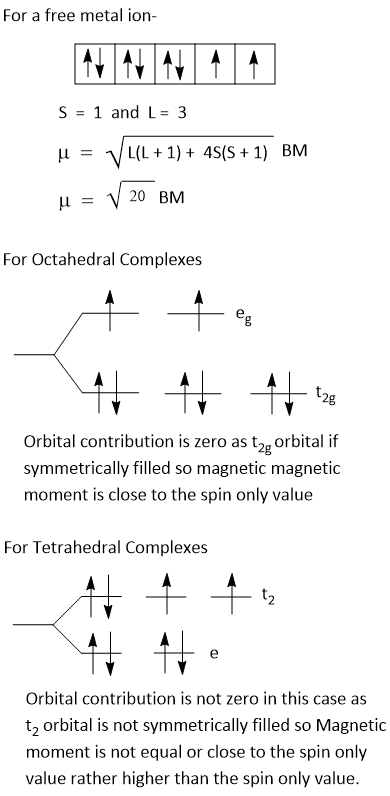
Orbital contribution to magnetic moment is only due to t2g or t2 rotation. However, if t2g or t2 are evenly occupied by electrons, as t2g3, t2g6, t23, t26, then orbital contribution to magnetic moment is fully quenched and in that case μeff-

Orbital contribution occurs only for t2g1egx, t2g2egx, t2g4egx, t2g5egx or for corresponding t2.
If ground term in crystal field is T, due to orbital contribution μeff is greater than μs. Thus, for d1, d4, d6, d7 weak field octahedral crystal field , d3, d4, d8, d9 in tetrahedral crystal field, d1, d2, d4, d5 in strong octahedral crystal field, there is orbital contribution and magnetic moment of these is greter than μs value.
Let's Consider a Ni(II) complex-
Electronic configuration is d8