Factors Affecting Magnitude of 10Dq | Δ
Crystal Field Splitting Energy
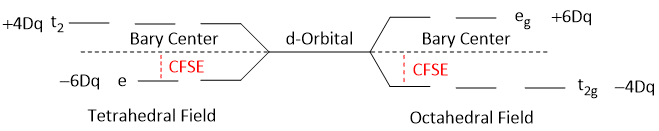
The energy difference between the Bary center and the lowest splitted d-orbitals is called Crystal Field Splitting Energy or Crystal Field Stabilization Energy (CFSE). In other words, the amount of energy by which a complex is stabilized in crystal field is called CFSE. Greater the CFSE, greater the stability of the complex.
Example- Calculation of CFSE of d4 Low Spin and d4 High Spin Complexes.
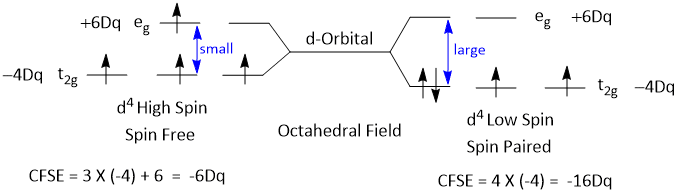
CFSE of d4 Low Spin complex is more than that of d4 High Spin. So, d4 Low Spin is more stable complex.
Crystal Field Splitting Parameters
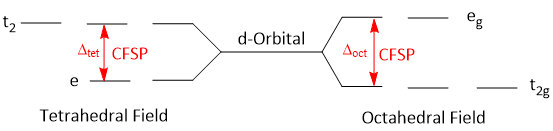
The energy difference between splitted d-orbitals i.e. t2g and eg in octahedral field and t2 and e in tetrahedral field is called Crystal Field Splitting Parameters.
It is denoted by 10Dq or Δ.
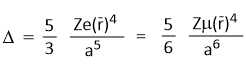
Where-
Z= Charge on the metal ion
e = Charge on the ligand
r = Average distance of d-orbitals charge density
a = Inter nuclear distance between metal ion and ligand
μ = Dipole moment of the ligand
Factors affecting magnitude of 10Dq | Δ
There are several factors that affect the magnitude of splitting of d-orbitals by the surrounding ligands. Some of which are mentioned below-1. Charge on the Metal Ion
2. Radius of the Metal Ion
3. Period Number of the Metal Ion
4. Charge or Diploe Moment of the Ligand
5. Donar-Acceptor property of the Ligand
6. Geometry of the complex
1. Charge on the metal ion
Since crystal field theory is based on the electrostatic model, the ionic charge on the central metal has a direct effect on the magnitude of 10Dq. In general, a metal ion with higher charge draws the ligands more closely, and hence produces more splitting than cation with lower charge. Thus 10Dq values of hexaquo complexes of Cr2+ and Cr3+ are 166.1 KJ/mol and 213.1 KJ/mol respectively. If ligand and geometry of the complexes are same, then magnitude of 10Dq increases by 1.5 times with an increase of one unit charge.
2. Radius of the Metal Ion
Magnitude of 10Dq is inversly proportional to radius of the metal ion i.e. larger the radius of the metal ion , lower the magnitude of 10Dq.
3. Period Number of the Metal Ion
Greater the extension of d-orbital electron density, greater is the value of 10Dq. 5d is more extended than 4d and 4d is more extended than 3d. In other words, the period number of the metal ion increases by one unit , the 10Dq value increses by about 30%.
4. Charge or Diploe Moment of the Ligand
Magnitude of 10Dq increases with increases the charge or diploe moment of the ligand.
5. Donar-Acceptor property of the Ligand
By forming π bonds, donar coordinated ligands like F−, Cl−, O−2 etc. decreases the 10Dq value. Whereas, acceptor coordinated ligands like CN−, CO etc. increases the 10Dq value.
6. Geometry of the complex
In octahedral complexes, the splitting of d orbitals is more than twice as strong as in tetrahedral complexes for the same metal ion and ligands. This difference in 10Dq value is because of two factors-
a. In octahedral complexes six ligands are involved while in tetrahedral only four; this results in 33% (i.e. 2/3rd) decrease in the field strength, provided the other factors remain the same.
b. In octahedral complexes, the ligands are situated exactly in the direction of dz2 and dx2−y2 orbitals while in tetrahedral complexes the ligands are not aimed at any of the d orbitals but exert more influence on the t2g orbitals than on the eg orbitals. In case of square planar complexes, the degree of splitting is more than in a tetrahedral field.
Pairing Energy
The energy required to force the two unpaired electrons in one orbital is called the pairing energy.
When more than one electrons are paired, P becomes the mean pairing energy. It may be obtained from the analysis of electronic spectra.
If Δo > P, it favours the low spin complexes.
If Δo < P, it favours the high spin complexes.
If Δo = P, high spin and low spin complexes are equalyy exist.
In general, for 4d and 5d series transition metal complexes, magnitude of Δo is greater than that of P. So, favours always low spin complexes
For 3d elements, a typical value of P is about 15,000 cm-1.
3d complexes are high spin with weak field ligands and low spin with strong field ligands.
High valent 3d complexes (e.g., Co3+ complexes) tend to be low spin (large Δo)
Test Your Knowledge
What will be the electronic configuration of d5 in terms of t2g and eg in an octahedral field when Δo < P, where P is the energy required for pairing of electrons in a single orbital ?
A. t2g5 eg0
B. t2g2 eg3
C. t2g3 eg2
D. t2g0 eg5
View Answer
Option C is correct answer.
When Δo < P the electrons prefer to occupy higher energy eg orbitals rather than pair up in the lower energy t2g orbitals. This results in a high-spin configuration.
For a d5 ion in an octahedral field under these conditions, the electronic configuration is t2g³ eg²
If the crystal field splitting energy (Δ) is less than the pairing energy (P), what type of complex is formed?
A. Low-spin complex
B. High-spin complex
C. Both high-spin and low-spin complexes
D. No complex is formed
View Answer
Option B is correct answer.
When Δ < P, electrons prefer to remain unpaired in higher energy orbitals, resulting in high-spin complexes
For which type of ligands is the pairing energy usually greater than the crystal field splitting energy?
A. Strong field ligands
B. Weak field ligands
C. Both strong and weak field ligands
D. None of the above
View Answer
Option B is correct answer.
With weak field ligands, Δ is small, so P > Δ, leading to high-spin complexes.
In a tetrahedral complex, which is always true regarding Δt (tetrahedral splitting) and P?
A. Δt > P
B. Δt < P
C. Δt = P
D. Δt and P are unrelated
View Answer
Option B is correct answer.
Tetrahedral complexes always have Δt less than P, so they always form high-spin complexes.
Which of the following statements is correct?
A. Strong field ligands lead to high-spin complexes
B. Weak field ligands lead to low-spin complexes
C. When Δ > P, electrons pair up in lower energy orbitals
D. When Δ < P, electrons pair up in lower energy orbitals
View Answer
Option C is correct answer.
Strong field ligands (large Δ) favor electron pairing in lower energy orbitals, resulting in low-spin complexes.
Also read
Crystal Field Theory
Splitting of d-Orbitals or Crystal Field Splitting
