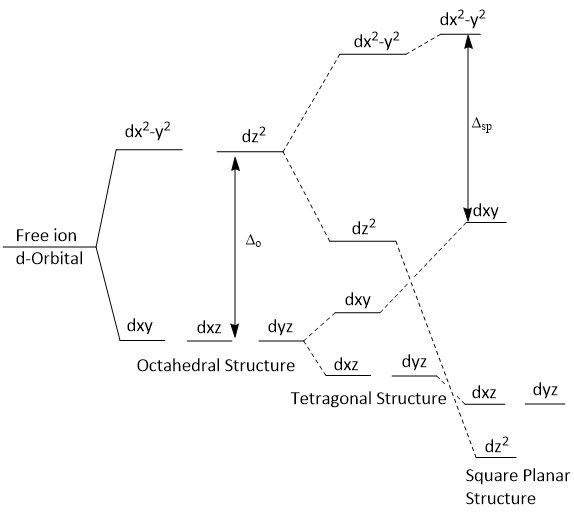
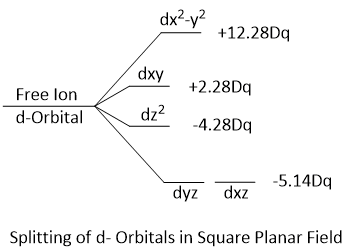
Splitting of d-Orbitals (Crystal Field Splitting)
Crystal Field Splitting
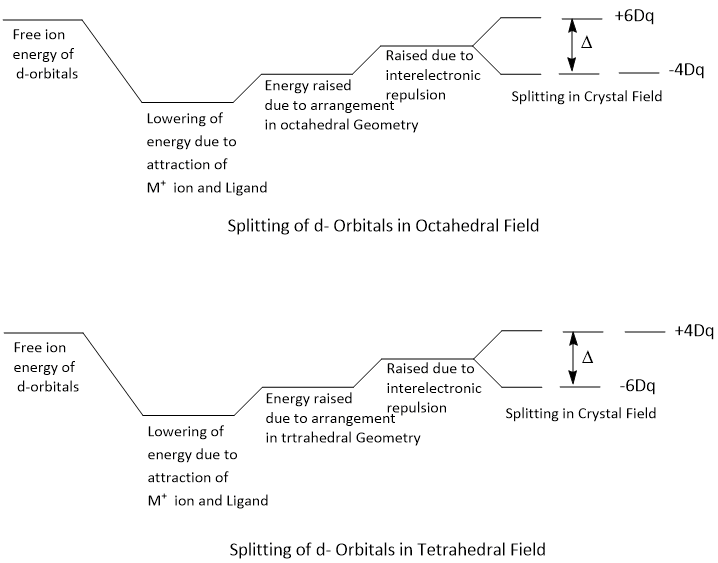
Crystal field splitting is the conversion of five degenerate d-orbitals of a metal ion into different sets of orbitals with varying energies in the presence of a crystal field of ligands.When positively charged metal ion and negatively charged ligand approach to each other, the energy of degenerate five d-orbitals is lowered in the beginning due to electrostatic attraction but consequently due to the arrangement of a particular geometry and also due to inter electronic repulsion between metal electron and ligand electron, the energy of degenerate d-orbitals is slightly raised to give the excited five fold degenerate d-orbitals. Till now, the degeneracy of d-orbitals is maintained.
The five fold degeneracy of d-orbitals is finally lost under influence of electrostatic field created by the interaction of metal and ligand. Therefore, those d-orbitals lying along the direction of approaching ligands are raised in energy and those d-orbitals lying away are lowered in energy.
Thus, five d-orbitals of the metals ion which are degenerate in absence of any ligand i.e. in gaseous state, split into groups of orbitals of different charges depending upon the electric field generated by ligands i.e. in solution or compounds. Therefore, the net result due to metal ligand interaction is the splitting of the degenerate d-orbitals of the metal into groups of orbitals having different energy. This splitting of d- orbitals is called crystal field splitting. Due to crystal field splitting, all d-orbitals are stabilised i.e. energy is lowered. However there is no change in the energy of the system due to the splitting if all five d-orbitals are equally occupied e.g. d0, d10 and d5 (high spin) systems.
The splitting of d-orbitals depends upon many factors like number of ligands, nature of ligands etc.
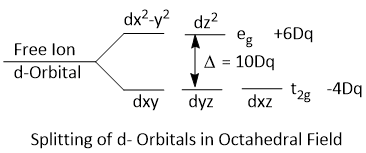
Splitting of d-Orbitals in Octahedral Field
In an octahedral complex, the metal ion is placed at the center of the octahedron and the six ligands are at the six corners. These six corners are directed along x, y and z axes as shown in figure.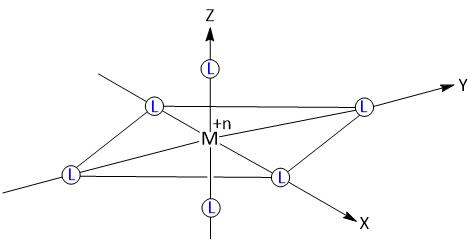
When all the six ligands are far away from the central metal atom, the five d-orbitals are degenerate as there is no effect of ligand electrostatic field. When the six ligands approach the central metal ion, the two d-orbitals (i.e. dx2−y2 and dz2) point directly towards the ligands and the three orbitals (i.e. dxy, dxz and dyz) point in between the path of the approaching ligands. Therefore, the dx2−y2 and dz2 orbitals will be more strongly repelled than dxy, dxz and dyz orbitals. Consequently, the energy of dx2−y2 and dz2 orbitals are much more than that of dxy, dxz and dyz orbitals. The two dx2−y2 and dz2 are designated as eg and the three dxy, dxz and dyz orbitalsa are designated as t2g.
The crystal field splitting in octahedral complexes is denoted by Δoct or Dq.
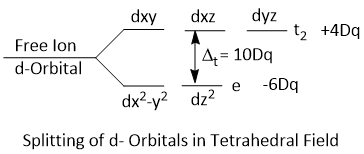
Splitting of d-Orbitals in Tetrahedral Field
A regular tetrahedral geometry is obtained when a metal cation is placed at the center of the cube and four ligands occupy the alternate corners of the cube as shown in figure.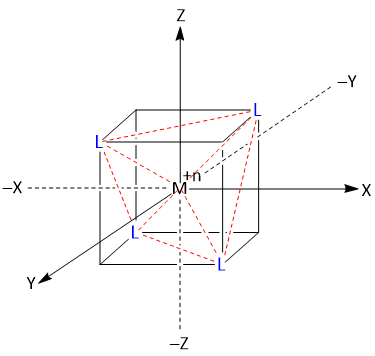
The x, y and z axes passed through the center of the faces of the cube and none of the ligands approach directly to any of the metal d-orbitals. Instead they all approach to some degree in between the metal d-orbitals.
The dxy, dxz and dyz orbitals are pointing close to the direction of approaching ligands and experience more repulsion while the dx2−y2 and dz2 orbitals are laying between the direction of approaching ligands and experience less repulsion. That means d-orbitals splitted into two groups. One group is 't2' (i.e. dxy, dxz and dyz) and the other group is 'e' (i.e. dx2−y2 and dz2). Thus, the energy of t2 orbitals increases much more than that of 'e' orbitals.
The crystal field splitting in tetrahedral complexes is denoted by Δtet or Dq.
Since, the d-orbitals are interacting indirectly with the ligands in tetrahedral field, the energy gap between t2 and e orbitals is about twice less than that in octahedral field.
Δtet = (4/9)Δoct
2Δtet = Δoct
This is also partly due to lesser number (i.e. 2/3 of octahedral) of ligands in tetrahedral fields. Hence, CFS favours octahedral complexes rather than tetrahedral complexes. Tetrahedral complexes are spin free (i.e. high spin) complexes as the energy gap is very small (i.e. less than pairing energy). Due to these reasons no pairing of electrons occurs in d3, d4, d5, d6 and d7 tetrahedral complexes. Therefore, the tetrahedral complexes of these configurations are always high spin whether the ligands are strong or weak.
Splitting of d-Orbitals in Square Planar Field
The splitting of d-orbitals in tetragonal and square planar complexes can be understood by gradually withdrawing two trans ligands lying along the Z-axis from an octahedral complex.As the ligands laying on the Z-axis are moved away, the ligands in the XY plane tend to approach the central metal ion more closely. As a result of this, the electrons in d-orbitals in the XY plane experience greater repulsion from the electrons of ligands in a tetragonal complex than in an octahedral complex. This causes an increase in the energy of d-orbitals in XY plane, i.e., increase in the energy of dry and day orbitals in tetragonal complexes compared to their energies in octahedral complexes.
Further, since the ligands lying on the Z-axis have been moved away, the electrons in the d-orbitals along the Z-axis as well as in the XZ and YZ planes experience relatively smaller repulsions from the electrons the ligands. This results in appreciable fall in the energy of dz2 orbital as well as dxz and dyz orbitals. This structure in which the two trans ligands are at larger distance as compared to the other four ligands in XY plane is called tetragonal structure.
As the trans ligands lying along the Z-axis drop out completely, square planar complex is formed. This is accompanied by further rise in the energies of dx3-y and dxz orbitals and a further fall in the energies of dz2, dxz and dyz orbitals. Crystal field splitting in the case of the square planar complex i indicated by Δsp.