Selection of Indicators for Acid Base Titration
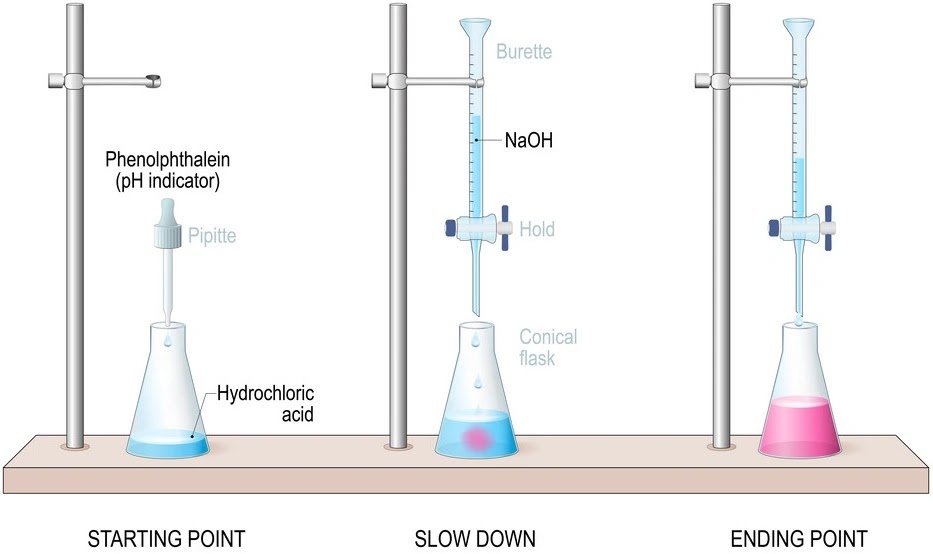
The acid-base indicator (is a dye that changes colour when pH changes) is usually an organic compound that is itself a weak acid or weak base. So, they have their own pH values. They change their colors within a definite pH range. For example Methylorange change its color at the pH range 3 - 4.5 and phenolphthalein change its color at the pH range 8 - 10. On the other hand pH at the equivalent point of acid-base react may not be 7 always. In some case it is greater than 7, less than 7 or in some case it is equal to 7 and it is decided by the nature of acid and base present in the reaction.
Read More
