Selection of Indicators for Acid Base Titration
Selection of Indicators for Acid Base Titration
The acid-base indicator (is a dye that changes colour when pH changes) is usually an organic compound that is itself a weak acid or weak base. So, they have their own pH values. They change their colors within a definite pH range. For example Methylorange change its color at the pH range 3 - 4.5 and phenolphthalein change its color at the pH range 8 - 10. On the other hand pH at the equivalent point of acid-base react may not be 7 always. In some case it is greater than 7, less than 7 or in some case it is equal to 7 and it is decided by the nature of acid and base present in the reaction.
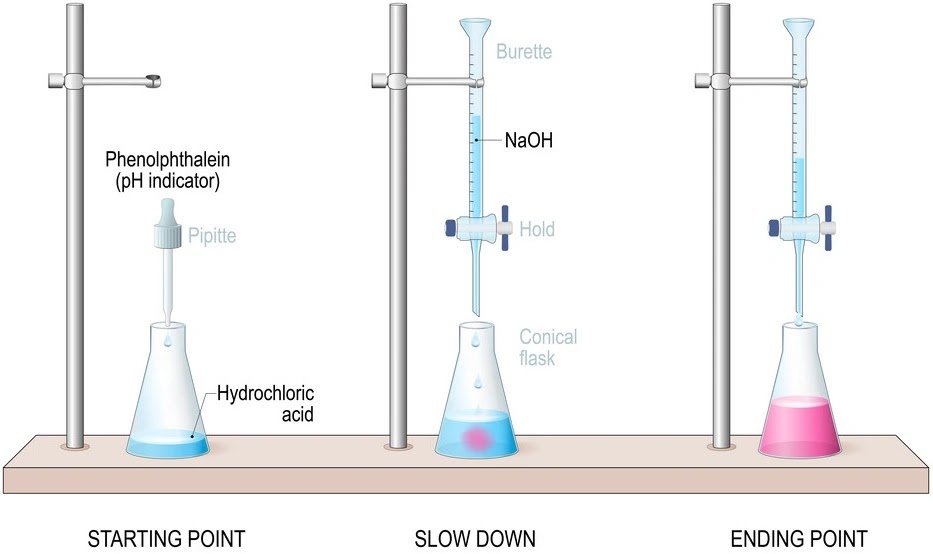
In order to determine the accurate end point (when the indicator changes colour during a titration) of acid-base titration, the pH indicator should be selected in such a way that the pH range for the color change of the indicator must coincide with the pH at the equivalent point (the amount of acid and of base is just sufficient to cause complete consumption of both the acid and the base. i.e. neither the acid nor the base is in excess or neither the acid nor the base is the limiting reagent) of reaction. Litmus is not used in titrations because the pH range over which it changes colour is too great (pH range is 5.0 - 8.0)
| Indicator Name | pH Range | Color Change |
|---|---|---|
| Methyl Orange | 3.1 - 4.4 | Red → Yellow |
| Bromothymol Blue | 6.0 - 7.6 | Yellow → Blue |
| Phenolphthalein | 8.3 -10.0 | Colourless → Pink |
1. Strong Acid Titration
HCl + NaOH → NaCl + H2O
The pH at this type of reaction is 7 (neutral) because the salt does not undergo hydrolysis with water. The pH change at the end of this type of titration is 3 - 10 approx. So, either methyl orange (3 - 4.5) or phenolphthalein (8 - 10) can be used to find the actual end point of this type of titration.
2. Strong Acid-Weak Base titration
HCl + NH4OH ⇋ NH4Cl + H2O
The pH at the equivalent point of this type of reaction is less than 7 (acidic) because the salt undergoes hydrolysis to give strong acid and weak base. The pH change at the end point of this type of titration is 3 - 7 approx. So, methyl orange (3 - 4.5) is good indicator for this type of titration.
3. Weak Acid-Strong Base titration
CH3COOH + NaOH ⇋ CH3COONa + H2O
The pH at the equivalent point of this type of reaction is more than 7 (basic) because the salt undergoes hydrolysis to give Strong base and weak acid. The pH change at the end point of this type of titration is 7 - 10 approx. The indicator used to find the end point of this type of titration should give the color change in basic range. Phenolphthalein is good indicator for this type of titration.
4. Weak Acid-Weak Base titration
CH3COOH + NH4OH ⇋ CH3COONH4 + H2O The pH at the equivalent point of this type of reaction may be either slightly greater than 7, less than 7 or equal to 7 and it is decided by the relative extent of ionization of Weak Acid and weak base produced after the hydrolysis of the salt. The pH change at the end point of this type of titration is not sharp and wide because the salt acts as a buffer. Because of no sharp and wide pH change at the end point of this indicator method of titration is not so accurate for weak acid and weak base titration.
