Phase Diagram of Lead –Silver system
Phase Diagram of Lead –Silver system
It is a two component system. The two metals are completely miscible in liquid state and does not form any compound. There is almost no effect of pressure on this system. The temperature composition (T-C) phase diagram is shown below-
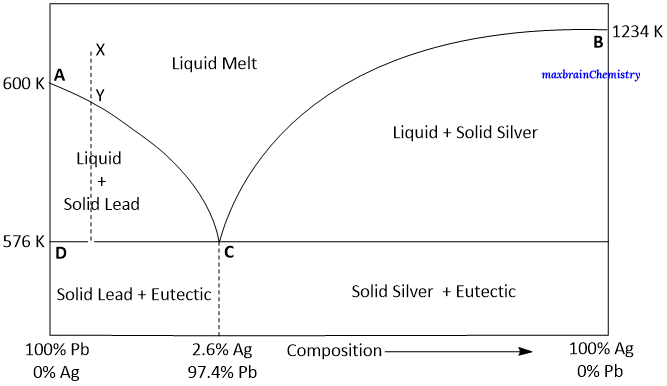
Lead –Silver system contains-
1. Curve AC
2. Curve BC
3. Eutectic Point C
4. Area
Curve AC
The curve AC is the freezing point curve of pure lead. The melting point of lead decreases gradually along the curve AC, with the continuous addition of silver. Thus, the curve AC is showing the effect of addition of silver on the melting point of pure lead. All along the curve AC two phases – solid lead and
liquid are in equilibrium.
Applying the Reduced Phase rule equation-
F = C – P + 1
or, F = 2 – 2 + 1
or, F = 1
or, F = 1
Thus, it is a univariant system.
Curve BC
Curve BC is the freezing point curve of pure silver and represents the effect of addition of pure lead on the melting point of pure silver. All along the curve BC two phases – solid silver and liquid are in equilibrium.
Applying the Reduced Phase rule equation-
F = C – P + 1
or, F = 2 – 2 + 1
or, F = 1
or, F = 1
Thus, it is a univariant system.
Point C
Point C is the eutectic point where solid silver, solid lead and their solution coexist. The curves AC and BC meet at point C. Since the experiment is carried out at constant pressure.
Applying the Reduced Phase rule equation-
F = C – P + 1
or, F = 2 – 3 + 1
or, F = 0
Thus, it is a non-variant or invariant system and the number of degree of freedom for the system at the eutectic point C is zero.
Areas
The area above the line ACB has a single phase (molten Pb+Ag).
Applying the Reduced Phase rule equation-
F = C – P + 1
or, F = 2 – 1 + 1
or, F = 2
Thus, the system is bivariant.
Both the temperature and composition have to be specified to define the system completely.
The area below the line AC ( solid Pb + liquid melt), below the line BC ( solid Ag +liquid melt) and below the eutectic point 'C' have two phases and the system is univariant.
Applying the Reduced Phase rule equation-
F = C – P + 1
or, F = 2 – 2 + 1
or, F = 1
Thus, the system is univariant.
Related Topics
Phase Diagram of Water System
Phase Diagram of KI-Water System
