Jablonski Diagram
Jablonski Diagram
Jablonski diagrams describe the electronic states of molecules, transitions and associated light emitting phenomena.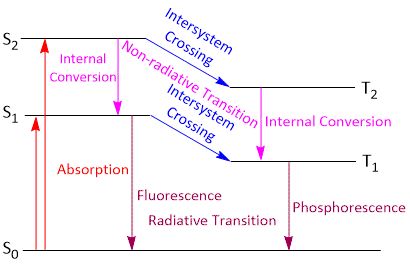
Jablonski diagram is a powerful tool for visualising the possible transitions that can occur after a molecule has been photoexcited. A typical Jablonski diagram is shown above.
The energy levels of a molecule are shown by the horizontal black lines. The naming of the electronic states is based on the spin angular momentum configuration of each state. Singlet states (a total spin angular momentum of zero) are denoted by S and triplet states (a total spin angular momentum of one) by T.
S0 is the singlet ground state of the molecule
S1 is the first excited singlet state
T1 is the first excited triplet state
Singlet excited state has higher energy than the corresponding triplet excited state. Accordingly, the energy sequence is-
ES1 > TS1
ES2 > TS2
ES3 > TS3
and so on...
On absorption on light photon, the electron of the absorbing molecule may jump from S0 to S1, S2 or S3 singlet excited state depending upon the light photon absorbed. For each singlet excited state (S1, S2, S3), there is a triplet excited state (T1, T2, T3). The molecule, whether in singlet or triplet excited state, is said to be activated.
The activated molecule rturns to the ground state by dissipating its energy through the radiative transitions, non-radiative transitions or chemical reactions.
Radiative Transitions
These transitions involve the return of the activated molecule from the singlet excited state (S1) and triplet excited state (T1) to the ground state (S0). Such transitions are accompanied by the emmision of radiation. Spectroscopically, the transition from S1 to S0 state is an allowed transition and occurs in about 10−8 second. The emission of radiation in this transition is called fluorescence. The transition from T1 to S0 state is rather slow since it is a forbidden transition. The emission of radiation in this transition is called phosphorescence. The life time of phosphorescence are much longer 10−8 second or greater since, the transition involves spin inversion which needs time for spin inversion.Both fluorescence and phosphorescence radiations are of shorter frequencies than the exciting light because some part of light energy absorbed by the molecules is dissipated in the form of heat during non-radiative transition.
Non-radiative Transitions
These transitions involves the return of the activated molecule from the higher excited states (S3, S2 or T3, T2) to the first excited state (S1 or T1). These transitions do not involve in the emmision of any radiations and are thus reffered to as non-radiative transition or radiationless transition. The energy of activated molecule is dissipiated in the form of heat through molecular collisions.The process is called internal conversion and occurs in less than about 10−11 second.The molecule also lose energy by another process called intersystem crossing. This process involves transitions between states of different spins i.e. diferent multiplicity (2s+1) as for example- from S2 to T2 or S1 to T1. These transitions are also non-radiative or radiationless. Spectroscopically, such transitions are forbidden. However, they do occur through at relatively slow rates.
Chemical Reactions
The activated molecule may also lose energy by undergoing chemical reaction. Since the molecule in singlet excited statereturns quickly to the ground state, it gets no chance to react chemically.However, The molecule in the triplet excited state, returns to the ground state comparatively slowly. This provide ample opportunity to the activated molecule to undergo chemical reaction. Thus, the molecule which undergoes chemical reaction is the one which is previously in a triplet excited state.
Share
