Effective Nuclear Charge
The effective nuclear charge (Zeff) is the nuclear charge felt by an electron when both the actual nuclear charge (Z) and the repulsive effects (shielding) of the other electrons are taken into account. In general, Zeff is given by-
Zeff = Z − S.
where S is called the shielding constant or screening constant.
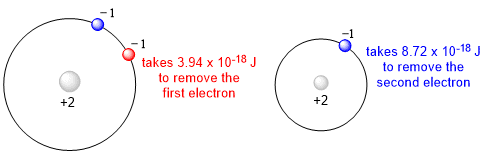
The shielding constant is greater than zero but smaller than Z. One way to illustrate how electrons in an atom shield one another is to consider the amounts of energy required to remove the two electrons from a helium atom. Experiments show that it takes 3.94 x 10-18 J to remove the first electron and 8.72 x 10-18J to remove the second electron. There is no shielding once the first electron is removed, so the second electron feels the full effect of the +2 nuclear charge.
Because the core electrons are, on average, closer to the nucleus than valence electrons, core electrons shield valence electrons much more than valence electrons shield one another. Consider the second-period elements from Li to Ne. Moving from left to right across the period, we find the number of core electrons (1s2) remains constant while the nuclear charge increases. However, because the added electron is a valence electron and valence electrons do not shield each other well, the net effect of moving across the period is a greater effective nuclear charge felt by the valence electrons, as shown here.
| Li | Be | B | C | N | O | F | Ne | |
|---|---|---|---|---|---|---|---|---|
| Z | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Z* | 1.28 | 1.91 | 2.42 | 3.14 | 3.83 | 4.54 | 5.10 | 5.76 |
The effective nuclear charge also increases as we go down a particular periodic group. However, because the valence electrons are now added to increasingly large shells as n increases, the electrostatic attraction between the nucleus and the valence electrons actually decreases.
Significance of Effective Nuclear Charge
Atomic Radii
Higher effective nuclear charge pulls the electron cloud closer to the nucleus, resulting in a smaller atomic radius.
Ionization Energy
Higher effective nuclear charge results in higher ionization energy as electrons are more strongly attracted to the nucleus.
Electron Affinity
Elements with higher effective nuclear charge tend to have higher electron affinities, as they more readily attract additional electrons.
Electronegativity
Higher effective nuclear charge generally corresponds to higher electronegativity, as the nucleus attract strongly the bonding electrons.
Shielding Effect
Effective nuclear charge helps to explain the shielding effect.
Spectroscopic Properties
Effective nuclear charge impacts the energy levels of electrons which affects the spectral lines an element emits or absorbs. This is used for identifying elements and compounds.
Source: General Chemistry: The Essential Concepts (6th ed.) By Raymond Chang and Jason Overby
Test Your Knowledge
Effective Nuclear Charge and Significance MCQs
Question 1 of
Correct: 0 / Attempted: 0
Test Completed!
You got 0 out of answers correct.
That's 0%
