Energy Dissipation in Excited Molecules: Radiative vs. Non-Radiative Processes
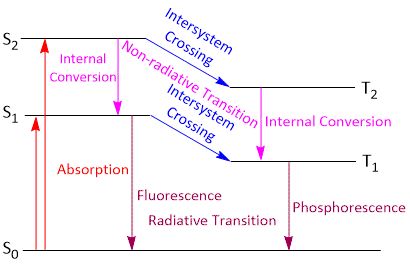
On absorption of a quantum of energy, the electron of the absorbing molecule may jump from So to S1, S2, S3... Sn. Singlet excited states depending upon the energy of the quantum absorbed, as shown in the Jablonski diagram.
For each singlet excited state S1, S2, S3 ... Sn, there is a corresponding triplet excited state T1, T2, T3 ... Tn. The molecule in singlet or triplet excited state is said to be activated.
Thus; Ao + hν → A*
Where, Ao and A* are the molecule in the ground state and excited state respectively.
The activated molecule returns to the ground state by dissipating its energy through the following types of processes:
- Non-Radiative Process
- Radiative Process
Non-Radiative Processes
These transitions involve the return of the activated molecule from the higher (activated states) excited state (S3 S2 or T3, T2) to the first excited state (S1 or T1). These transitions do not involve the emission of any radiations and are thus referred to as non radiative or radiation less transitions. The energy of the activated molecule is dissipated in the form of heat through molecular collisions. The process is called internal conversion (IC) and occurs in less than about 10-11 second.
The other type of non-radioactive transition, in which transition take place higher singlet state to another singlet state at this time it's shown same spine multiplicity. This process called internal conversion (IC).
When the molecules loss energy by another process called Inter system crossing (ISC). In this process involves transitions between lowest excited singlet state (S1, S2) to corresponding lowest triplet state,( T1,T2) i.e. S1 to T1 or S2 or T2. This process transition between states of different spins shown different multiplicity and Spectroscopically, forbidden .Such transitions are also non-radiative or radiation less. However, they do occur though at relatively slow rates.
It is clear from Figure, this transition take place near the cross over point of two potential energy curves. If any molecules jump from singlet state to triplet state cannot return back to singlet state. So it reached to triplet state vibrational level ν' = 0. From this stage it return back singlet So state through producing Phosphorescence.
Also read Kasha's Rule
Radiative Processes
A radiative transition is one in which the energy is released as a photon. The nature of the emission depends on the nature of the initial and final states and the route to the excited state. These transitions involve the return of the activated molecule from the singlet excited state S1 and triplet excited state T1 to S0. These transitions are of two types:
- When molecules produce fluorescence
- When Molecules produce Phosphorescence
When Molecules Produce Fluorescence
When by the absorbing radiation transition take place from So to S1, S2 then excited molecules due to collision S1 to S2 lose some energy. In this way some parts of vibrational energy by internal conversion process change to heat. By releasing energy in the form of heat molecules reached to the ν' = 0 state. From this point in S1 excited state the molecules may return back to ground state So. When the molecules return back to ground state So it emits radiations. It is called fluorescence or fluorescence spectrum. Spectroscopically, the transition from S1 to So is allowed transition and occurs in about 10-8 second.
It is clear from figure that the substance absorbs high energy radiation to become excited and after 10-8 second they emit radiation having shorter wavelength. These substances are called fluorescence matters and the process of emission of radiation is known as fluorescence.
When Molecules Produce Phosphorescence
When active molecules return back to ground state So from first triplet excited state. It is clear that when the transition from lowest vibrational energy level ν' = 0 to ground state So is Spectroscopically forbidden because the selection rule ΔS is not applicable, but this transition occur in slower rate than allowed electronic transition.
The emission of this transition is called Phosphorescenc. The time period of Phosphorescenc are much longer being in the order of 10-3 second or more, since the transition involves spin inversion which need time for its occurrence.
In both fluorescent and phosphorescent radiations are of shorter frequencies than the exciting radiation. This is because; some part of the absorbed energy by the molecules is dissipated in the form of heat during non-radiative transitions.
