Vapour Pressure | Factors Affecting the Vapour Pressure
Vapour Pressure
We know that when a liquid placed in an open vessel evaporates completely. If, however, the liquid is allowed to evaporate in a closed vessel evaporation occurs, but after sometime the level of the liquid does not change any further and become constant.
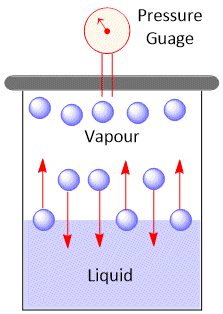
Let us understand how does it happen. In the closed vessel, the molecules evaporating from the liquid surface are confined to a limited space. These molecules may collide among themselves or with the molecules of air and some of them may start moving towards the surface of the liquid and enter into it. This is known as condensation. In the beginning, rate of evaporation is greater than the rate of condensation. But as more and more molecules accumulate in the space above the liquid, rate of condensation gradually increases. After some time, rate of evaporation becomes equal to the rate of condensation and an equilibrium state is reached. The number of molecules in the vapour above the liquid becomes constant. These molecules exert certain pressure over the surface of the liquid. This pressure is known as equilibrium vapour pressure or saturated vapour pressure or simply as vapour pressure.
The pressure exerted by vapour on the surface of the liquid is called vapour pressure of that liquid at constant temperature. The vapour pressure of a liquid has a characteristic value at a given temperature.
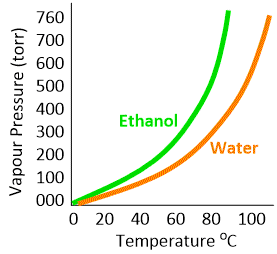
The vapour pressures of various liquids differ considerably, depending upon the identity of the liquid with its particular intermolecular forces. Thus ethanol having weaker hydrogen bonding than water, evaporates faster than water. Hence we expect that ethanol will have higher vapour pressure than water at a given temperature.
Factors Affecting the Vapour Pressure
Two important factors that affect the vapour pressure of a liquid are given below-1. Nature of the Liquid
2. Temperature
Effect of Nature of the Liquid on Vapour Pressure
If the intermolecular force of attractions in the liquid are weak, the molecules can easily leave the liquid and come into the vapour phase and hence the vapour pressure becomes higher. Example- vapour pressure of ether, acetone and benzene etc. is higher that that of water at the same temperature.
Effect of Temperature on Vapour Pressure
If the temperature of the liquid is increased, the vapour pressure will increase because at higher temperature more molecules in the liquid will have larger kinetic energy and will break away from the liquid surface. Therefore, the concentration of vapour molecules will increase before the equilibrium is re-established. Also, at higher temperature, the average kinetic energy of the vapour molecules will increase. Both vapour concentration and kinetic energy are proportional to temperature. Therefore, any increase of temperature will result in the increase of vapour pressure. From the experimental curves shown in Figure it is clear that for both ethyl alcohol and water, the vapour pressure rises with increase of temperature.
1. Explain vapour pressure of pure solvent is more than that of solution.
2. When vapour pressure of a liquid is equal to the atmospheric pressure then what will happen to the liquid?
3. Which of the following factors does not affect the vapor pressure of a liquid?
A. Concentration of solutionsB. Volume of the liquid
C. Temperature
D. Intermolecular forces
