Types of Magnetic Behavior
Classfication of Magnetic Behavior
The origin of magnetism lies in the orbital and spin motions of electrons and how the electrons interact with one another. The best way to introduce the different types of magnetism is to describe how materials respond to magnetic fields. In magnetic field some substances are repelled and some are attracted. The substances which are repelled from the magnetic field are called diamagnetic and which are attacted in magnetic field are called paramagnetic. Some substances are attracted very strongly towards magnetic field are called ferromagnetic. Antiferromagnetism and ferromagnetism are special case of paramagnetism.The magnetic behavior of substances can be classified into the following five major groups-
1. Diamagnetism
2. Paramagnetism
3. Ferromagnetism
4. Ferrimagnetism
5. Antiferromagnetism
Diamagnetism
Diamagnetic properties arise from the realignment of the electron paths under the influence of an external magnetic field. In diamagnetic substances all the electrons are paired so there is no net permanent magnetic moment per atom. It is independent of magnetic field strength and its magnetic susceptibility is negative. Diamagnetic substances are slightly repelled by a magnetic field and do not retain the magnetic properties when the external field is removed.Most elements in the periodic table, including copper, silver, and gold, are diamagnetic in nature.
Paramagnetism
Paramagnetic properties are due to the presence of some unpaired electrons, and from the realignment of the electron paths caused by the external magnetic field. In the presence of an external magnetic field, paramagnetic substances tend to move from a region of a weak to a strong magnetic field and do not retain the magnetic properties when the external field is removed. In a paramagnetic substance, the individual atoms possess a dipole moment, which wen placed in a magnetic field, interact with one another, and get spontaneously aligned in a common direction, which results in its magnetization. Paramagnetic substances have a small, positive susceptibility to magnetic fields.Magnesium, Molybdenum, Lithium, and Tantalum are examples of paramagnetic substances.
Ferromagnetism
Ferromagnetic properties are arises due to interaction of magnetic dipoles parallel to each other (↑↑). In a ferromagnetic substance, the individual atoms possess a dipole moment, similar to a paramagnetic substance and have a large positive susceptibility to an external magnetic field. When a ferromagnetic substances are placed in an external magnetic field, all unpaired electrons at different lattice sites orient in one particular direction i.e. parallel to each other and give rise to a large number of unpaired electrons in the lattice and get strongly magnetized and are able to retain their magnetic properties after the external field is removed. Also, they tend to move from a region of weak to the region of a strong magnetic field and get strongly attracted to a magnet.Iron, Cobalt and Nickel are examples of ferromagnetic materials.
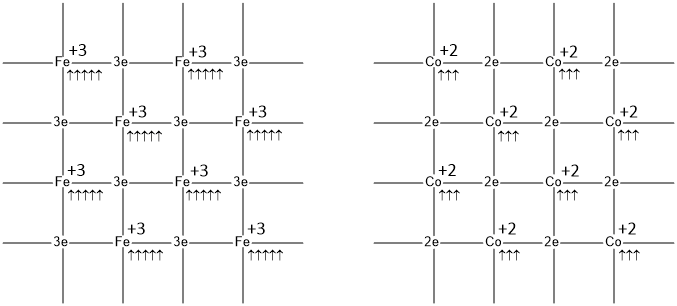
Ferromagnetic propert is greater for greater number number of electrons. So the order of ferromagnetism of the above metals is-
Fe > Co > Ni
Ferrimagnetism
It is a special case of anti-ferromagnetism. Partial antiferromagnetism occurs in such substances. The unpaire electrons in the substance are aligned in parallel and anti-parallel directions in unequal numbers. They are weakly attracted by magnetic field as compared to ferromagnetic substances. On heating, ferrimagnetic substances lose their ferrimagnetism and become paramagnetic.Magnetite like Fe3O4 and ferrites like MgFe2O4 and ZnFe2O4 are examples of ferrimagnetic substance.
Fe3O4 has spinel structure as-
Fe(II) Fe(III) Fe(III)
(oct) (oct) (tet)
↑↑↑↑ ↑↑↑↑↑ ↓↓↓↓↓
O−2 has closed packed structure and has octahedral holes and tetrahedral holes.
There are two octahedral holes per tertahedral hole. The unpaired electrons of Fe(III) at octahedral and tetrahedral sites orient in opposite direction and so paramagnetism is only due to Fe(II) electrons.
Anti-Ferromagnetism
Anti-ferromagnetism arises due to anti-parallel (opposite) orientation of unpaired electrons at different lattice sites. These opposite magnetic sites have equal magnetic moments which are canceled out (since they are in opposite directions). This makes the net moment of the substance zero.Some substances contain unpaired electrons but in the magnetic field they behave as diamagnetic substances as if all the electrons are paired up. Anti-ferromagnetism is depend upon the field strength (H) applied and its susceptibility is positive.
The Neel temperature is the temperature at which an antiferromagnetic material begins to be converted into a paramagnetic material. At this temperature, the thermal energy provided is large enough to break down the alignment of magnetic sites present in the material.
[Cu(CH3COO)2H2O]2, [Cr(CH3COO)2H2O]2, Mn2(CO)10 and MnO are antiferromagnetic substance.
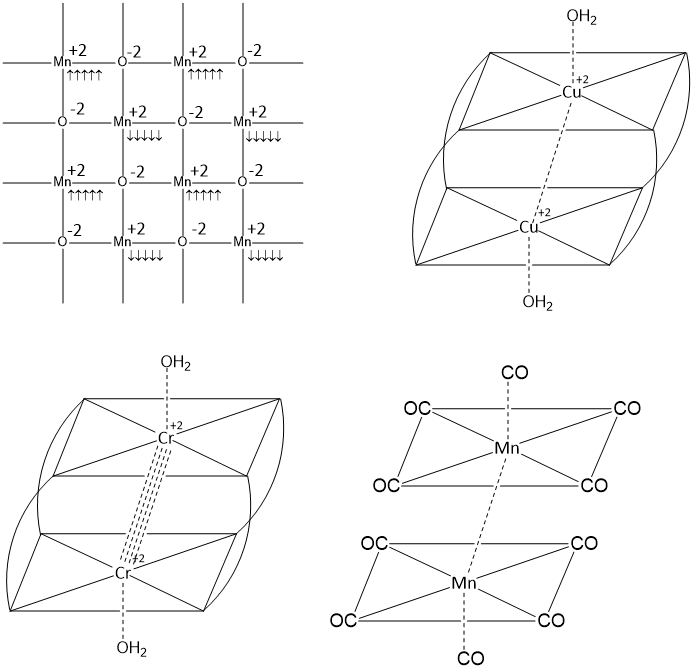
At high temperature, the Metal-Metal bond breaks and the substance becomes paramagnetic.
Share
