Nernst's Equation or Relation between emf and Activity of a Cell
Nernst's Equation or Relation between emf and Activity of a Cell
The Nernst Equation was given by German Chemist Walther Hermann Nernst. Nernst Equation is used to determine the cell potential of an electrochemical cell at a given temperature, pressure and reactant concentration. The cell potential of electrochemical cells can be determined with the help of the Nernst Equation even under non - standard conditions. It gives the relationship between the cell potential and the reaction quotient of a reaction and accurately determines equilibrium constants (including solubility constants).This equation can be applied both to the potentials of individual electrodes and the potential differences across a pair of half-cells. However, it is generally more convenient to apply the Nernst equation to one electrode at a time.
A galvanic cell is the cell which generates electricity from energy change accompanying a chemical reaction. The emf of such cell arises due to the reaction occuring in the cell. If the following reaction occurs in a reversible chemical cell-

Equation 4 is Nernst equation in terms of activity and Equation 5 and 6 is Nernst equation in terms of concentration
Applications of Nernst's Equation
The Nernst equation can be used to calculate-1. Emf of an electrochemical cell.
2. Standard electrode potentials.
3. Single electrode reduction or oxidation potential at any conditions.
4. The pH of solutions and solubility of sparingly soluble salts.
5. Unknown ionic concentrations.
6. Determination of equilibrium constant for cell reaction
7. Ionization constant and degree of dissociation of weak acid or weak base
Relation Between Free Energy Change and Equilibrium Constant
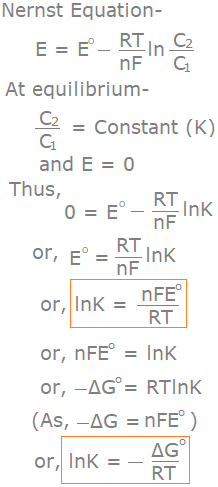
Relation Between emf and Free Energy Change of a Cell
A reactant possesses a specific internal energy which is called free energy.Change in free energy-
−ΔG = Gfinal − Ginitial
As the result of cell reaction occuring in a galvanic cell, the change in free energy is equal to the product of emf(E) of the cell and the quantity of electricity (nF) that has passed through the cell.
i.e. −ΔG = nEF
where- 'n' is the valency of the element
'F' is 1 Faraday = 96500 Coulombs.
Thus, if the passage of the 'n' Faraday of electricity liberates one gram atom of the element.
i.e. −ΔG = nEF
Thus,
1. If ΔG is negative, FE is positive or vice versa and cell reaction will occur spontaneously.
2. If ΔG is positive, FE is negative and cell reaction will not possible.
3. If ΔG is zero, and E = 0, the cell reaction will be in equilibrium and hence, the reaction will be reversible.
