Grignard Reagent
Grignard Reagent
R — Mg — X

Grignard reagents are organomagnesium compounds represented as RMgX, where R is an alkyl or aryl group and X is a halogen, named after the French chemist Victor Grignard.
Grignard reagents are highly reactive and are generally used for forming carbon-carbon bonds. Grignard reagents are prepared by reacting an alkyl or aryl halide with magnesium metal in an anhydrous ether solvent or tetrahydrofuran (THF). Water and air rapidly destroy the reagent by protonolysis or oxidation, so it cannot be use.
R-X + Mg → R-Mg-X
Alkyl halide
Ar-X + Mg → Ar-Mg-X
Aryl halide
Reactions of Grignard Reagents
As Nucleophiles
Grignard reagents react with number of carbonyl derivatives. Some important reactions are given below-
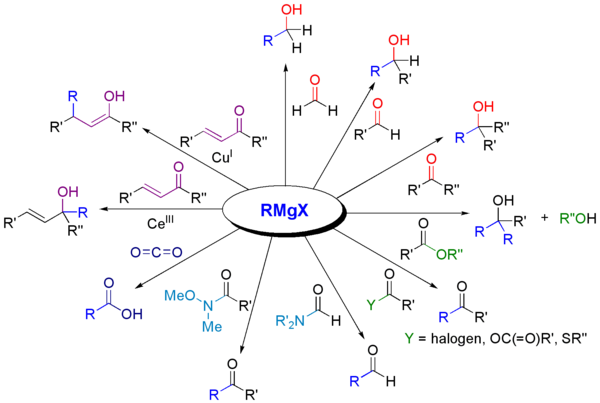
Grignard reagents also react with many carbonyl-like compounds and other electrophiles.

Reactions as a Base
Grignard reagents are basic and react with alcohols, phenols, etc. to give alkanes and alkoxides (ROMgBr).
R-OH + RMgX → RO−Mg−X + R-H
H-OH + RMgX → RO−Mg−X + R-H
Alkylation of Metals and Metalloids
Like organolithium compounds, Grignard reagents are also useful for forming carbon–heteroatom bonds.

Grignard reagents react with many metal-based electrophiles. For example, they undergo transmetallation with cadmium chloride (CdCl2) to give dialkylcadmium:
2 RMgX + CdCl2 → R2Cd + 2 Mg(X)Cl
Coupling with Organic Halides
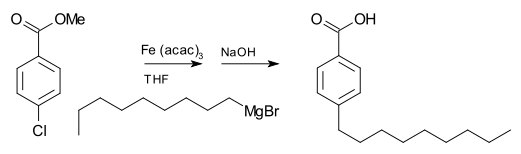
Grignard reagents generally does not react with organic halides, in contrast with their high reactivity with other main group halides. In the presence of metal catalysts, however, Grignard reagents participate in C-C coupling reactions.
For example, nonylmagnesium bromide reacts with methyl p-chlorobenzoate to give p-nonylbenzoic acid, in the presence of Tris(acetylacetonato)iron(III) (Fe(acac)3), after workup with NaOH to hydrolyze the ester. Without the Fe(acac)3, the Grignard reagent would attack the ester group over the aryl halide.
Oxidation
Treatment of a Grignard reagent with oxygen gives the magnesium organoperoxide. Hydrolysis of this material yields hydroperoxides or alcohol. These reactions involve radical intermediates.

Elimination
In the Boord olefin synthesis, the addition of magnesium to certain β-haloethers results in an elimination reaction to the alkene. This reaction can limit the utility of Grignard reactions.

Sources: Wikipedia
