Glass Electrode for pH Measurement
Glass Electrode for pH Measurement
Glass electrode is a type of ion selective electrode used mainly for the measurement of the pH of a solution because it is not affected by oxidising or reducing agents and is not easily poisoned.The half-cell reaction of the glass electrode is represented as-
H+ (unknown) | glass membrane | 0.1 N HCl | AgCl(s) – Ag
Glass electrode is based on the principle that which a glass memberane separates two solutions differing in pH, a potential difference is found to exist between the two surface of the glass which varies with the difference of pH of the two solutions.
Glass electrode consists of a thin walled glass bulb made up of special type of glass (Soda lime glass)
Soda lime glass is composed of about 70% silica (silicon dioxide), 15% soda (sodium oxide), and 9% lime (calcium oxide)
having low melting point and high electrical conductivity. It is filled with normal hydrochloric acid solution saturated with quinhydrone or in contact with Ag/AgCl electrode. The platinum wire dipping in the electrolyte passes out of the glass tube at G. The bulb is placed in the solution of which the pH is to be measured and which is contained in the beaker B. The potential is measured against a standard calomel electrode C.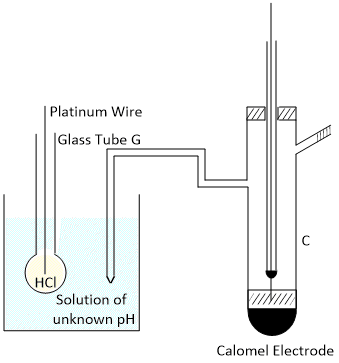
--> An ordinary potentiometer can not be used to measure the voltage of such a cell because of the high resistance of the glass membrane and for this reason valve electrometers which require very little current for their operation are made use of.
The potential EG of the glass electrode is given by the relation-
EG = EoG − (2.303RT/F)log10[H+]
EG = EoG − 0.0951 log10[H+] at 25°C
EG = EoG − 0.0951 pH
The value of EoG is obtained by calibrating the apparatus by means of buffer solutions of known pH value. Since the potential of the calomel electrode is known that of the glass electrode can be easily calculated from the observed emf of the complete cell and the value of pH obtained with the help of the relationship given above.
The glass electrode gives very good result for pH range 0 to 9. For high alkalinity, high accuracy is not obtained.
Advantages of Glass Electrode for pH Measurement
The common advantages of the application of glass electrodes in pH measurement are-1. The ion selective glass electrode gives a fairly accurate measurement of pH within an error of ± 0.05.
2. The equilibrium of the electrode is quickly attained. It cannot be influenced by any gas, oxidizing, or reducing agents.
3. The range of pH which can be measured is 2 to 9. However, new glasses have been developed with which the pH range can be extended up to 13 or 14.
4. Glass electrodes are used for a long time for pH measurement if carefully handled and stored in distilled water.
Limitations of Glass Electrode for pH Measurement
Limitations of glass electrodes in pH measurement are-1. The glass membrane though it is very thin, it offers high resistance. Therefore ordinary potentiometers cannot be used; hence it is necessary to use electronic potentiometers.
2. This electrode cannot be used to determine the pHabove 12.
Quinhydrone Electrode for pH Measurement
Share
