Electronic Transition
Electronic transition refers to the movement of an electron from one energy level to another within an atom or molecule. This process is a fundamental aspect of the interactions between light and matter and plays a crucial role in various fields such as chemistry, physics, and biology. The excitation of molecule by absorption of radiation in UV visible region involves promotion of electrons from bonding and non-bonding to antibonding orbital. There are σ and 𝜋 bonding orbitals associated with σ* and 𝜋* antibonding orbitals respectively. Non bonding orbitals are not associated with antibonding orbitals because non-bonding or lone pair of electrons does not form bond but attached with them.
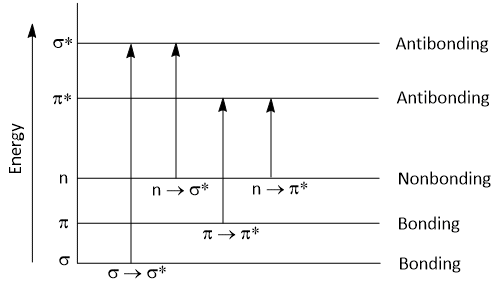
Following electronic transitions occur in UV visible region-
σ → σ*, n → σ*, 𝜋 → 𝜋* and n → 𝜋*
Types of Electronic Transition
According to molecular orbital theory, when a molecule is excited by the absorption of energy (UV or visible light), its electrons are promoted from a bonding orbital to antibonding orbital.
The antibonding orbital which is associated with the excitation of σ-bonding orbital electron is called σ* antibonding orbital. So, σ to σ* transition takes place when σ electron is promoted to σ* orbital. This transition is denoted as σ → σ* transition.
When non-bonding (n) electron promoted to an antibonding (σ*) orbital, then it is denoted as n → σ* transition.
Similarly, When 𝜋 electron promoted to an antibonding (𝜋*) orbital, then it is denoted as 𝜋 → 𝜋* transition.
When non-bonding (n) electron promoted to an antibonding (𝜋*) orbital, then it is denoted as n → 𝜋* transition.
The energy required for this transition is in the following order-
σ → σ* > n → σ* > 𝜋 → 𝜋* > n → 𝜋*
σ → σ* Transition
This type of transition takes place in saturated compounds having no hetero atoms. It is a high energy process because σ bond is generally very strong. The molecule in which all the bonds are sigma bonds do not show absorption in the normal UV- region 200-400nm. Usually this transition requires radiation of very short wavelength below 200nm. The usual spectroscopic technique cannot be used below 200nm, since oxygen present in air begins to absorb strongly. To study such high energy transitions below 200nm, the entire path length must be evacuated. Thus, the region below 200nm is commonly called vacuum ultraviolet region. The excitation of σ bond electron to σ* antibonding orbital occurs with net retention of electronic spin. It is called excited singlet state which may, in turn, gets converted to excited triplet state.
n→ σ* transition
This type of transition takes place in saturated compounds containing one hetero atom with unshared pair of electrons (n electrons). Some compounds undergoing this type of transitions are saturated halides, alcohols, ethers, aldehydes, ketones, amines etc. Such transitions require comparatively less energy than that required for σ → σ* transitions.
In saturated alkyl halides, the energy required for such a transition decreases with the increase in the size of the halogen atom (or decrease in the electronegativity of the atom). Hydrogen bonding shifts the UV absorptions to shorter wave lengths.
𝜋 → 𝜋* Transition (K-Band)
This type of transition occurs in the unsaturated centres of the molecule; i.e., in compounds containing double or triple bonds and also in aromatics. The excitation of 𝜋 electron requires smaller energy and hence, transition of this type occurs at longer wavelength. An electron of a double bond is excited to 𝜋* orbital. For example, alkenes, alkynes, carbonyl compounds, cyanides, azo compounds etc. show 𝜋 → 𝜋* transition.
n → 𝜋* Transition (R-Band)
In this type of transition, an electron of unshared electron pair on hetero atom gets excited to 𝜋* antibonding orbital. This type of transition requires least amount of energy out of all the transitions above and hence occurs at longer wavelengths. Saturated aldehyde show both types of transitions, i.e. low energy n → 𝜋* and high energy 𝜋 → 𝜋*. Absorption occuring at lower wavelength is usually intense. In saturated carbonyl compounds, two types of transitions take place-
High energy transitions
n → σ* (intense)
𝜋 → 𝜋* (intense)
Low energy transition
n → 𝜋* (weak).
In carbonyl compounds, the shift in the absorption depends upon the polarity of the solvent.
| Transition Type | Electron Movement | Energy Range | Intensity | Occurs In |
|---|---|---|---|---|
| σ → σ* | Bonding σ to antibonding σ* | High (UV region) | Strong | Saturated hydrocarbons |
| 𝜋 → 𝜋* | Bonding 𝜋 to antibonding 𝜋* | Moderate (UV-visible) | Strong | Alkenes, aromatics |
| n → σ* | Non-bonding lone pair to antibonding σ* | High (vacuum UV) | Weak | Molecules with lone pairs |
| n → 𝜋* | Non-bonding lone pair to antibonding 𝜋* | Lower than 𝜋→𝜋* | Weak | Carbonyls, amines |
Undergraduate Level Questions
1. Define electronic transition in a molecule.
View Answer
Electronic transition refers to the movement of an electron from one energy level (orbital) to another in an atom or molecule, usually caused by absorption or emission of energy, such as UV or visible light.
2. List and briefly describe the four main types of electronic transitions observed in UV-Visible spectroscopy.
View Answer
- σ → σ*: Electron moves from bonding sigma orbital to antibonding sigma orbital (high energy).
- n → σ*: Electron moves from non-bonding orbital to antibonding sigma orbital.
- π → π*: Electron moves from bonding pi orbital to antibonding pi orbital.
- n → π*: Electron moves from non-bonding orbital to antibonding pi orbital (lowest energy).
3. Explain the difference between σ → σ* and π → π* electronic transitions. Which requires higher energy and why?
View Answer
σ → σ* transitions involve electrons in strong sigma bonds, requiring more energy and occurring in the vacuum UV region. π → π* transitions involve electrons in weaker pi bonds and happen at lower energies, usually visible UV region.
4. What kind of electronic transition occurs when a non-bonding electron is excited to a π* orbital? Mention typical functional groups where this occurs.
View Answer
This is an n → π* transition, common in carbonyl groups, amines, and other molecules with lone pairs.
5. Arrange the following transitions in order of decreasing energy requirement: n → π*, π → π*, n → σ*, σ → σ*.
View Answer
σ → σ* > n → σ* > π → π* > n → π*
6. Why is the σ → σ* transition usually observed below 200 nm in the vacuum UV region?
View Answer
Because σ bonds are very strong, requiring high energy (short wavelength) radiation to excite electrons; wavelengths below 200 nm fall in vacuum UV, which usually requires evacuated experimental setups.
7. Describe how hydrogen bonding affects the n → σ* transition in saturated alkyl halides.
View Answer
Hydrogen bonding shifts the absorption of n → σ* transitions toward shorter wavelengths (blue shift) due to changes in electron density around involved atoms.
Postgraduate Level Questions
1. Using molecular orbital theory, explain the formation of antibonding orbitals and their role in electronic transitions.
View Answer
Antibonding orbitals form by out-of-phase combination of atomic orbitals resulting in higher energy orbitals with nodes. Electron excitation from bonding or non-bonding orbitals to antibonding orbitals constitutes electronic transitions that weaken bonds and alter molecular properties.
2. Discuss the role of electronic spin states in the σ → σ* transition and the formation of singlet and triplet excited states.
View Answer
During σ → σ* transitions, electron spin is conserved resulting in singlet excited states. Intersystem crossing can lead to triplet states with parallel spin electrons, which have longer lifetimes and distinct photophysical behaviors.
3. Analyze the solvent effect on the n → π* electronic transition in carbonyl compounds and explain the shifts in absorption spectra.
View Answer
Polar solvents stabilize the non-bonding electron orbital more so than the π* antibonding orbital, causing bathochromic (red) or hypsochromic (blue) shifts in the absorption peaks of n → π* transitions depending on solvent polarity.
4. Describe the experimental challenges associated with studying σ → σ* transitions and the significance of vacuum UV spectroscopy.
View Answer
σ → σ* transitions require high-energy UV radiation below 200 nm which is absorbed by atmospheric oxygen. Therefore, these studies need evacuated or inert-gas atmospheres and specialized vacuum UV instrumentation for accurate measurement.
5. Compare and contrast the electronic transitions involving charge transfer with typical σ, π, and n electron transitions.
View Answer
Charge transfer transitions involve movement of electrons between different metal and ligand orbitals and often result in intense broad absorptions at visible wavelengths unlike localized σ, π, or n transitions, which usually have sharper absorptions in UV regions.
6. Propose a UV-Vis spectroscopic experiment to differentiate between π → π* and n → π* transitions in a compound containing both functional groups.
View Answer
Measure the UV-Vis absorbance in solvents of varying polarity. The n → π* transition peak will be more sensitive to solvent polarity and generally weaker in intensity, while π → π* peak is stronger and less affected by solvent change, enabling differentiation.
7. How can molecular vibrations and rotations affect the electronic transition spectra of polyatomic molecules?
View Answer
Coupling of vibrational and rotational states with electronic transitions leads to vibrational fine structure and broadening in electronic spectra. Franck-Condon factors explain variations in intensities based on overlap of vibrational wavefunctions in the initial and final states.
Scientific References
-
Caricato, M., Karl, M., & Jacquemin, D. (2010). Electronic transition energies: A study of the performance of a large range of single reference density functional and wave function methods on valence and Rydberg states compared to experiment. Journal of Chemical Theory and Computation, 6(10), 3701–3714.
https://doi.org/10.1021/ct9005129 -
Liu, Y., Song, M., & Hammes-Schiffer, S. (2019). Electronic transitions of molecules: Vibrating Lewis structures. Chemical Science, 10(29), 7093–7103.
https://doi.org/10.1039/C9SC02534K -
Masood, T. B., Singh, A., & Hegde, G. (2021). Visual analysis of electronic densities and transitions in molecules. IEEE Visualization Conference.
https://vgl.csa.iisc.ac.in/pdf/pub/MasoodVisualAnalysisElectronicTransitionEuroVis2021.pdf -
Head-Gordon, M., Rico, R. J., Oumi, M., & Lee, T. J. (1995). Analysis of electronic transitions as the difference of electron densities. The Journal of Physical Chemistry, 99(1), 14261–14270.
https://doi.org/10.1021/j100039a012
