Electrical Double Layer and Zeta Potential
The electrical double layer and zeta potential are fundamental concepts in colloid and interface science, playing a crucial role in understanding the behavior of particles and surfaces in various systems. The electrical double layer is the region of space near a charged surface where potential difference exists due to the presence of ions. It consists of two layers, the inner Helmholtz layer and the outer Helmholtz layer. The inner Helmholtz layer is a layer of ions attracted to the charged surface, which creates an electric field, and the outer Helmholtz layer is a layer of ions attracted to the inner Helmholtz layer, forming a counter-charge layer.
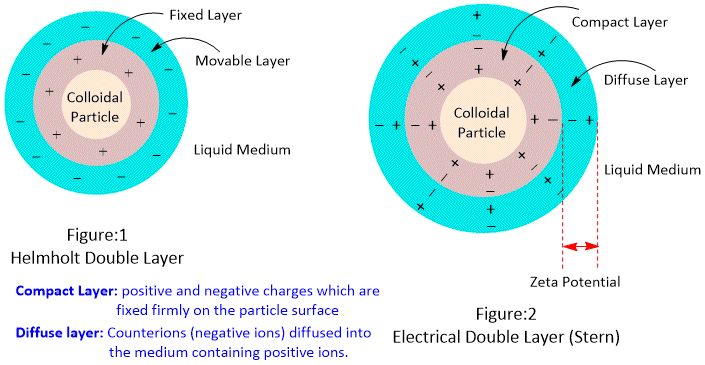
The formation of the electrical double layer can be explained by the Gouy-Chapman-Stern theory, which states that when a charged surface is placed in an electrolyte solution, ions from the solution are attracted to the surface and form a compact layer of ions. This layer of ions is pushed back by the repulsive forces between ions, creating a diffuse layer. The combination of the compact and diffuse layers forms the electrical double layer is reffered to as Stern Double layer. The thickness of this layer depends on various factors such as the ionic strength of the solution, surface charge density, and ion size. Gouy (1909) calculated the thickness (d) of diffused double layer by applying Debye-Huckel theory.
Zeta potential is the potential difference between the surface of the charged particle and the surrounding fluid. It is an important parameter to measure the surface charge of colloidal particles and is also used to predict the stability and behavior of colloidal systems. The zeta potential is affected by factors such as pH, temperature, and the presence of other ions in the solution.
Read More
